The MINT Team is working to develop novel technologies that will advance peripheral vascular and cerebrovascular therapies.
Our Vascular Technologies

Vascular Graft

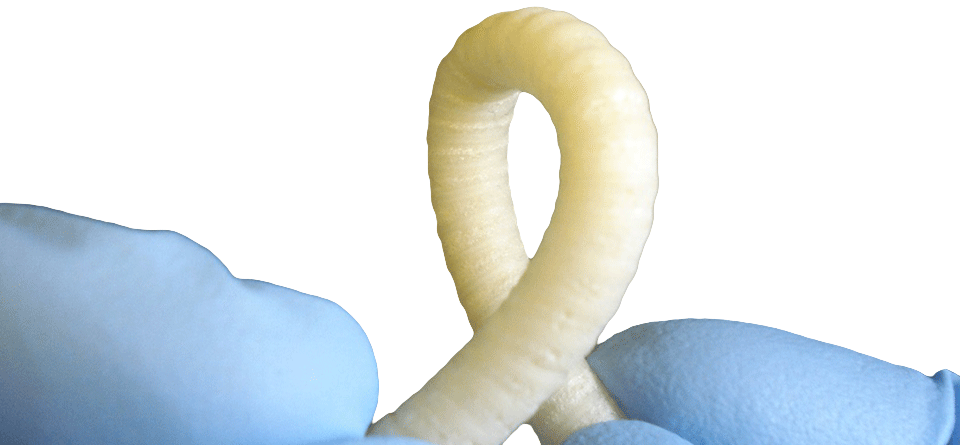
- Through a dynamic 5-year collaboration, MINT and Oxford Biomaterials (“OBM”, Oxford, UK) have jointly developed a novel vascular graft that addresses the most significant needs in vascular surgery
- To support joint development and commercialization efforts, MINT and OBM plan to form Vascular Silk Technologies Ltd (VST), a startup company focused on accelerating the VST pipeline of products to market and clinical use worldwide.
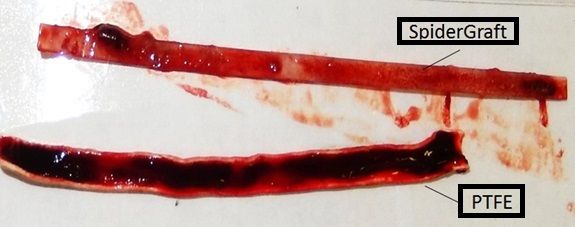
- VST's first product would be SpiderGraft, a revolutionary dialysis access graft that supports life-saving renal replacement therapy for more than 1.6M patients (and growing) worldwide.
- SpiderGraft shows excellent thrombo-resistance, minimal blood leakage after puncture and mechanical properties closer to native vessels when compared to current commercial grafts. With these properties, SpiderGraft could significantly improve clinical outcomes and eliminate more than $150M of costs from the US healthcare system each year in dialysis applications alone.
- As a platform technology, SpiderGraft will also generate significant clinical and economic value in the large and growing fields of peripheral vascular grafts, and vascular patches, endovascular devices, and coronary artery applications.
Unmet Clinical Need
- 1.6M+ people are on dialysis worldwide, with increasingly prevalent ESRD pre-conditions Diabetes and Hypertension continuing to drive future dialysis demand
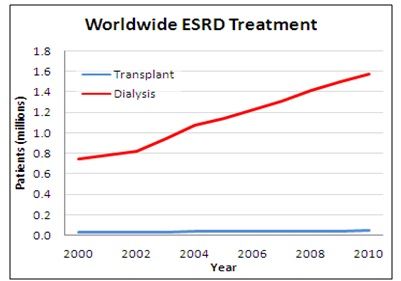
- All dialysis patients require vascular access sites through which blood can circulate to a dialyzer, and all access modalities present challenges:
- Catheters cause infection, central venous stenosis (more than 2.3 cases of sepsis per patient year)
- Native fistulas fail to mature (in 25% of cases, fistulas will never be able to support dialysis)
- Current Grafts fail to remain patent over time (50% of the time current dialysis grafts fail within one year)
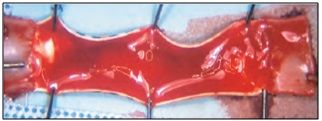
Heavily clotted PTFE graft segment
- More than 33% of the time, grafts remain the most advantageous access method for patients – therefore, a more effective dialysis graft is urgently needed to serve this patient population
- VST’s goal has been to develop a novel graft that significantly improves graft performance beyond that of currently available grafts.
Solution and Study Results

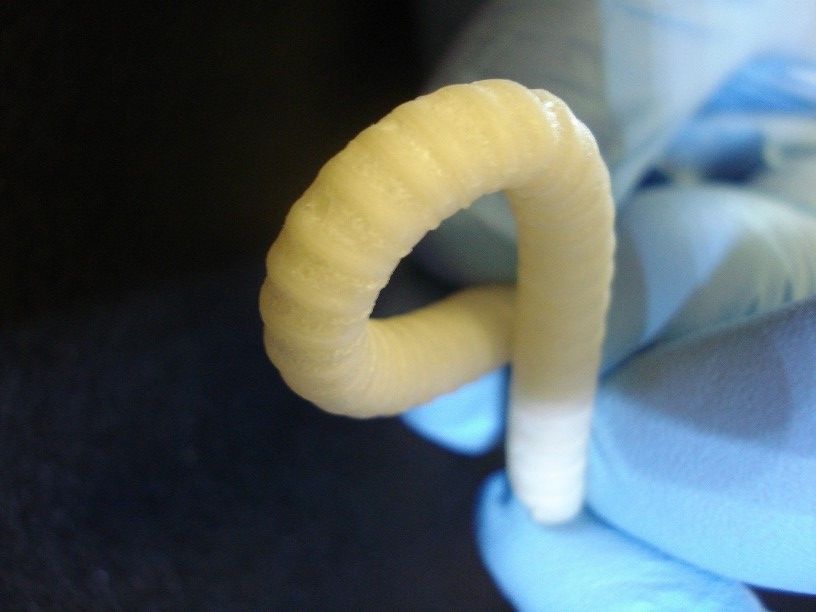
SpiderGraft anastomosis with pig artery
- SpiderGraft has enhanced hemocompatibility: study results suggest blood will not clot along its surface and the graft will be more resistant to thrombus formation, ensuring SpiderGraft remains open to blood flow. Current PTFE grafts clot at least 3x faster in vitro and generate 4x more thrombus in vivo.
Thrombus accumulation on SpiderGraft and PTFE in a canine study.
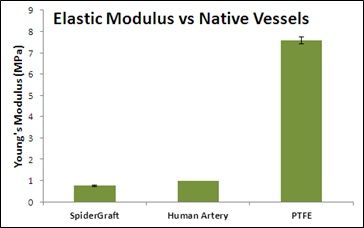
- SpiderGraft is more biomimetic: Its structure and mechanical properties more closely resemble those of human vessels, suggesting it will reduce trauma to native vessels, resultant vessel narrowing, and restricted blood flow. Current PTFE grafts are 8x stiffer than human vessels, potentially causing vascular tissue reaction, stenosis, and graft failure.
- SpiderGraft minimizes blood leakage: SpiderGraft could reduce blood leaking after cannulation, enabling SpiderGraft to be cannulated sooner after implantation and thereby reducing the need for emergency catheter-based dialysis access. Leak reduction could also reduce perigraft hematoma risks and therefore reduce access site infection compared to grafts commercially available today. Current PTFE grafts permit a larger defect after cannulation and allow fluid to leak at a rate 7x faster than SpiderGraft.
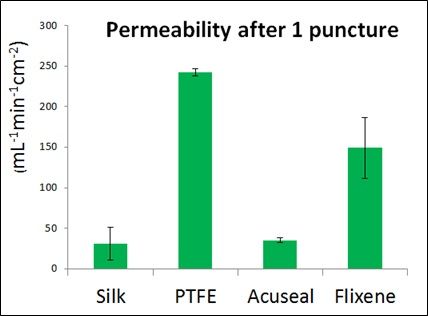
Graft permeability at 120mmHg. Acuseal is a Gore product marketed as an early-cannulation dialysis access graft.
SpiderGraft is designed to promote tissue integration: Its porous wall was engineered to encourage surrounding soft tissues and capillaries to tightly integrate with SpiderGraft, which would also reduce risks of perigraft hematoma and infection. Current PTFE grafts are non-porous, are not closely incorporated by tissue, and in 15% of cases become infected.
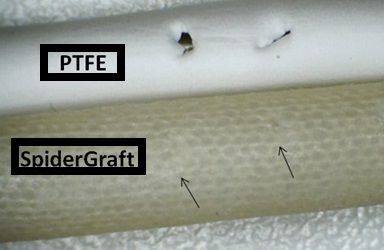
High-resolution photograph showing defects after cannulation with a 16G dialysis needle. Note the large defects in the PTFE material and almost indistinguishable puncture sites in SpiderGraft.